Description
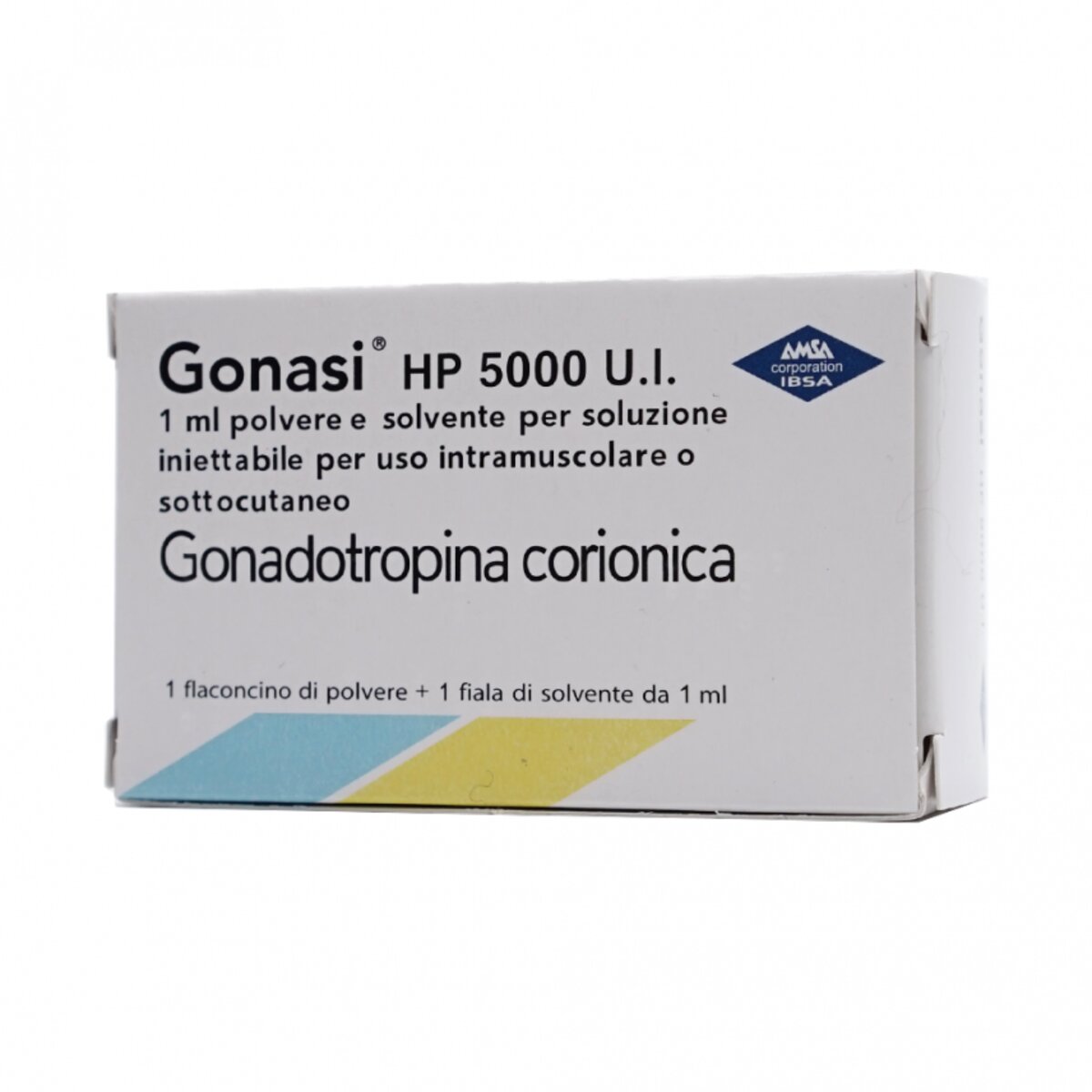
Gonasi Injection – Human Chorionic Gonadotropin Injection
Meet Gonasi Injection, the injectable Human Chorionic Gonadotropin (HCG) that can help with a variety of female and male reproductive issues. In women, it can be used to treat primary and secondary amenorrhea, ovarian hypoplasia, menometrorragia, recurrent miscarriage, threatened abortion, anovulatory infertility, and sterility ovogenesi deficient.
For men, it can be used to help with too little development of the sexual glands, delayed puberty, or problems with sperm formation. So whatever your reproductive issue may be, Gonasi Injection is here to help!
Gonasi Injection is available as 2000iu/ml and 5000iu/ml
Gonasi Injection Reviews
After using Gonasi Injection, it’s helpful to let others know about your experience. Reviews of an item help other users know that medicines received have helped the condition it is claimed for, how well the treatment worked or any issues to be aware of. We invite our users to leave a review of both their treatment and of the service provided. Click on the reviews tab to see if there has been feedback on this item.
What is the price of Gonasi Injection?
The price of Gonasi Injection starts from £84.50
Where can you buy Gonasi Injection?
You can buy Gonasi Injection at Dock Pharmacy Essex UK, UK Online Pharmacy.
Can you buy Gonasi Injection Over the counter?
Gonasi Injection is not available to buy over the counter. You need a prescription to buy Gonasi Injection
Product Details
Product Information
If signs of precocious puberty due to induction of androgen secretion promoted by chorionic gonadotropin, you must discontinue therapy.Because, in fact, androgens can cause fluid retention, chorionic gonadotrophin should be used with caution in patients with epilepsy, asthma or heart or kidney disease. The joint use of chorionic gonadotropin (HCG) with human menopausal gonadotropin (HMG) should assume complete knowledge of the contraindications, precautions for use and possible side reactions, the main ones are: ovarian enlargement, ascites with or withoutpain, pleural reaction, ruptured ovarian cyst resulting in hemoperitoneum, multiple births, arterial thromboembolism. Although not been no reported case of viral contamination associated with administration of gonadotropin extracted from human urine, the risk of transmission of pathogens known or unknown can not be totally excluded. Keep out of reach and sight of children. Have been reported headaches, fatigue, irritability, restlessness, depression, edema, precocious puberty, gynecomastia. Reactions may rarely occur in the place of painful injections. Following the administration of chorionic gonadotrophin in women undergoing ovulation induction treatments can cause multiple pregnancies and the occurrence of ovarian hyperstimulation syndrome which can be prevented through careful monitoring of treatments. This syndrome is mainly characterized by ovarian enlargement accompanied by pelvic pain, nausea, vomiting, weight gain and in severe cases, but rare, accumulation of fluid in the abdomen and thorax as well as more serious thromboembolic complications.
GONASI HP 5000 IU / 1 ml powder and solvent for solution for injection for intramuscular or subcutaneous
Each vial of powder contains:
Active Ingredient: Chorionic Gonadotrophin 5000 IU
PHARMACEUTICAL FORM 03.0 – Home Page
Powder and solvent for solution for injection
04.0 CLINICAL – Home Page
4.1 Therapeutic indications – Home Page
In children: cryptorchidism, hypogonadism, hypogonadism eunuchoidism.
In women: primary and secondary amenorrhea, ovarian hypoplasia, menometrorragia, recurrent miscarriage, threatened abortion, anovulatory infertility, sterility ovogenesi deficient.
In humans: azoospermia, oligoastenospermia, asthenospermia.
4.2 Posology and method of administration – Home Page
Guideline can be adopted the following scheme.
In women:
Amenorrhea, primary and secondary cycles anovulari: if an employee Deficit pituitary gonadotrophic secretion or lack of response to gonadal pituitary gonadotropins, obtained the optimal level of estrogen: 5000-10000 IUGonasi of HP.
Ovarian hypoplasia: 3-5 doses of 500-1000 U.I. HP Gonasi of the month.
Menometrorragie: 500-1000 U.I. every other day until termination of Gonasi HP bleeding, then 500 to 1,000 IU once a week to normalize the consecutive menstruation.
Recurrent miscarriage: 5,000 U.I. Gonasi of HP on alternate days during the first 3 months of pregnancy. Then for another 2 months, 1,000 U.I. every other day.
Threatened abortion: intervene promptly with 5,000 IU HP also Gonasi of 2 times per day until the threat of abortion. To follow the administration of 1,000 U.I. every 3 days.
Sterility deficient ovogenesi: after stimulation with human menopausal gonadotropin (HMG) injected at 24 hours after administration of 1-2 ampoules of HMG Gonasi HP 5000 IU waves lead to ovulation.
In humans:
Cryptorchidism: 250 – 500 – 1000 U.I. HP Gonasi of 2 or 3 times a week for periods of 40 days. Repeat the treatment after 30 days of suspension.
Hypogonadism: 125 – 250 – 500 U.I. of Gonasi HP 3 times a week.
Azoospermia, oligoastenospermia: 500 U.I.Gonasi of HP every other day for 3-4 months.
Asthenospermia: 1,000 to 2,000 U.I. Gonasi of HP every 4 days to 3 months.
Method of administration
The HP Gonasi must be injected intramuscularly or subcutaneously. The solution obtained by combining the solvent to dry powder should be used immediately after preparation.
3.4 Contraindications – Home Page
Precocious puberty. Hypertrophy or pituitary tumors, ovarian carcinoma, prostate or other androgen-dependent tumors, tumors of the testes. In the presence of other endocrine disorders (eg hypothyroidism, adrenocortical deficiency, hyperprolactinemia) must first be established adequate therapy. Primary testicular or ovarian failure, the absence of the uterus, early menopause, tubal occlusion (unless the patient is subjected to in vitro fertilization programs). Thrombophlebitis in the active phase. Hypersensitivity to the active substance or to any of the excipients.
4.4 Special warnings and precautions for use – Top
If signs of precocious puberty due to induction of androgen secretion promoted by chorionic gonadotropin, you must discontinue therapy.Because, in fact, androgens can cause fluid retention, chorionic gonadotrophin should be used with caution in patients with epilepsy, asthma or heart or kidney disease.
The joint use of chorionic gonadotropin (HCG) with human menopausal gonadotropin (HMG) should assume complete knowledge of the contraindications, precautions for use and possible side reactions, the main ones are: ovarian enlargement, ascites with or withoutpain, pleural reaction, ruptured ovarian cyst resulting in hemoperitoneum, multiple births, arterial thromboembolism.
Although not been no reported case of viral contamination associated with administration of gonadotropin extracted from human urine, the risk of transmission of pathogens known or unknown can not be totally excluded.
Keep out of reach and sight of children.
4.5 Interactions – Home Page
Avoid concomitant use of chorionic gonadotropin with high doses of corticosteroids.
6.4 Pregnancy and lactation – Home Page
As the hormone human placental origin is not contraindicated in pregnant women.
There are recognized indications of HCG coincident with breastfeeding.
4.7 Effects on ability to drive and use machines – Home Page
The product does not affect the ability to drive and use machines.
4.8 Undesirable Effects – Home Page
Have been reported headaches, fatigue, irritability, restlessness, depression, edema, precocious puberty, gynecomastia.
Reactions may rarely occur in the place of painful injections.
Following the administration of chorionic gonadotrophin in women undergoing ovulation induction treatments can cause multiple pregnancies and the occurrence of ovarian hyperstimulation syndrome which can be prevented through careful monitoring of treatments. This syndrome is mainly characterized by ovarian enlargement accompanied by pelvic pain, nausea, vomiting, weight gain and in severe cases, but rare, accumulation of fluid in the abdomen and thorax as well as more serious thromboembolic complications.
4.9 Overdose – Home Page
An overdose of chorionic gonadotropin in prepubertal children can lead to premature puberty and stunted growth.
05.0 Pharmacological Properties – Home Page
1.5 Pharmacodynamic Properties – Home Page
Pharmacotherapeutic group: Chorionic Gonadotropin
The HP Gonasi contains human chorionic gonadotropin obtained from the urine of pregnant women, collected between 60 ° – 90 ° day of pregnancy. The product obtained is further purified to obtain a title of 5000 IU / mg.
The chorionic gonadotropin extracted from the urine of pregnant women, in the Gonasi HP, has a biological action similar to that of luteinizing hormone, pituitary increto, which manifests itself in man by stimulation of Leydig cells in women with ‘induction of ovulation, maturation of the ovarian follicle sequentially.
Androgenic stimulation by human chorionic gonadotropin leading to the development of secondary sex characteristics and stimulate testicular descent when no anatomical impediment coexist. In women during the normal menstrual cycle causes the secretion of follicular estrogen, ovulation and then the secretion by the corpus luteum.
Chorionic gonadotropin is metabolized in the liver and excreted in the urine almost entirely.
06.0 Pharmaceutical – Home Page
6.1 Excipients – Home Page
Vial of powder: Lactose.
Solvent ampoule: sodium chloride, water for injections.
6.2 Special precautions for storage – Top
Store at temperatures not exceeding +25 ° C, in the original package to repair the product from light.
6.3 Nature and contents of the pack – Home Page
GONASI HP 5000 U.I. / 1 ml powder and solvent for solution for injection for intramuscular or subcutaneous
1 vial of powder + 1 ampoule of 1 ml
 +44 (0) 1375 846 316
+44 (0) 1375 846 316